University of Birmingham, Cooper Mass Spectrometry Group
What is the focus of your lab’s research?
Our research focuses on in situ analysis of intact proteins from biological substrates. We combine ambient surface techniques and ion mobility spectrometry with high resolution mass spectrometry.
We are particularly interested in native ambient mass spectrometry, in which folded proteins, protein assemblies and protein complexes are sampled directly from thin tissue sections. Native ambient mass spectrometry, such as liquid extraction surface analysis (LESA), is integrated with mass spectrometry imaging to provide simultaneous spatial and structural information.
We also apply LESA for the analysis of intact but unfolded proteins from a range of substrates including living microbial colonies growing on agar and other solid substrates, dried blood spots and tissue sections. The combination of LESA, ion mobility spectrometry and mass spectrometry enables the detection of hundreds of proteins.
Why did you incorporate the TriVersa NanoMate® into your laboratory
Initially, we purchased the TriVersa NanoMate® for direct infusion and coupling to LC and we still use the equipment for that purpose. Advion Interchim Scientific’s chip technology has revolutionized nanospray as far as ease of use. The ESI Chip™ is robust and bypasses any problems with non-uniformity. It allows us to move simply to the next nozzle if there is an issue with spray. The spray sensing capability is very clever and necessary for our overnight runs. More recently, we have used the LESA capability of the TriVersa NanoMate® for our in situ analyses of proteins in tissue, dried blood spots and microbial colonies.
To whom would you recommend the TriVersa NanoMate® for their research?
I would recommend the TriVersa NanoMate® to anyone with a mass spectrometer who uses nanoelectrospray.
Do you have any publications or presentations using the TriVersa NanoMate®?
Publication Highlight
Liquid Extraction Surface Analysis Mass Spectrometry of ESKAPE Pathogens
Havlikova et al. J Am Soc Mass Spec. 2021
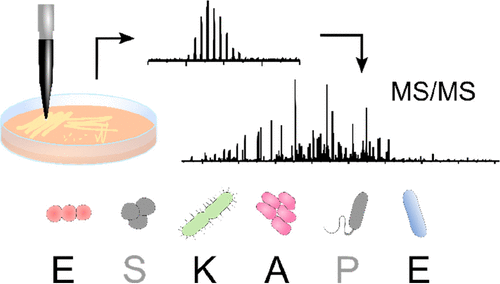
Top-down LESA MS/MS was used for protein identification in four ESKAPE pathogens as well as E. faecalis V583 and a clinical isolate of A. baumannii.
Other Publications:
- Hale et al. Native mass spectrometry imaging and in situ top-down identification of intact proteins directly from tissue. J Am Soc Mass Spec. DOI: 10.1021/jasms.0c00226
- Havlikova et al. Direct identification of bacterial and human proteins from infected wounds in living 3D skin models. Sci Rep. DOI: 10.1038/s41598-020-68233-6
- Haque et al. Self-incompatibility triggers irreversible oxidative modification of proteins in incompatible pollen. Plant Physiology. DOI: 10.1104/pp.20.00066
- Sisley et al. LESA cyclic ion mobility mass spectrometry of intact proteins from thin tissue sections. Anal. Chem. DOI: 10.1021/acs.analchem.9b05169
- Hale et al. Native LESA TWIMS-MSI: Spatial, conformational, and mass analysis of proteins and protein complexes. J Am Soc Mass Spec. DOI: 10.1021/jasms.9b00122
- Kocurek et al. Electroporation and mass spectrometry: A new paradigm for in situ analysis of intact proteins from living yeast colonies. Analytical Chemistry/ DOI: 10.1021/acs.analchem.9b04365
- Griffiths et al. Comprehensive LESA mass spectrometry imaging of intact proteins by integration of cylindrical FAIMS. Analytical Chemistry. DOI: 10.1021/acs.analchem.9b05124
- Havlikova et al. Quantitative imaging of proteins in tissue by stable isotope labeled mimetic liquid extraction surface analysis mass spectrometry. Analytical Chemistry. DOI: 10.1021/acs.analchem.9b04148
- Griffiths et al. LESA MS imaging of heat-preserved and frozen tissue: Benefit of multistep static FAIMS. Analytical Chemistry. DOI: 10.1021/acs.analchem.8b02739
- Rosting et al. High field asymmetric waveform ion mobility spectrometry in nontargeted bottom-up proteomics of dried blood spots. J Proteom Res. DOI: 10.1021/acs.jproteome.7b00746
- Sarsby et al. Liquid extraction surface analysis mass spectrometry coupled with field asymmetric waveform ion mobility spectrometry for analysis of intact proteins from biological substrates. Analytical Chemistry. DOI: 10.1021/acs.analchem.5b01151
- Griffiths et al. Liquid extraction surface analysis field asymmetric waveform ion mobility spectrometry mass spectrometry for the analysis of dried blood spots. Analyst. DOI: 10.1039/C5AN00933B
- Sarsby et al. Top-down and bottom-up identification of proteins by liquid extraction surface analysis mass spectrometry of healthy and diseased human liver tissue. J Am Soc Mass Spec. DOI:10.1007/s13361-014-0967-z
- Randall et al. Direct analysis of intact proteins from Escherichia coli colonies by liquid extraction surface analysis mass spectrometry. Analytical Chemistry. DOI: 10.1021/ac503349d
- Edwards et al. Compound heterozygotes and beta‐thalassemia: Top‐down mass spectrometry for detection of hemoglobinopathies. PROTEOMICS. DOI: 10.1002/pmic.201300316
- Martin et al. Dried blood spot proteomics: Surface extraction of endogenous proteins coupled with automated sample preparation and mass spectrometry. J Am Soc Mass Spec. DOI: 10.1007/s13361-013-0658-1
- Edwards et al. Hemoglobin variant analysis via direct surface sampling of dried blood spots coupled with high-resolution mass spectrometry. Analytical Chemistry. DOI: 10.1021/ac1030804

