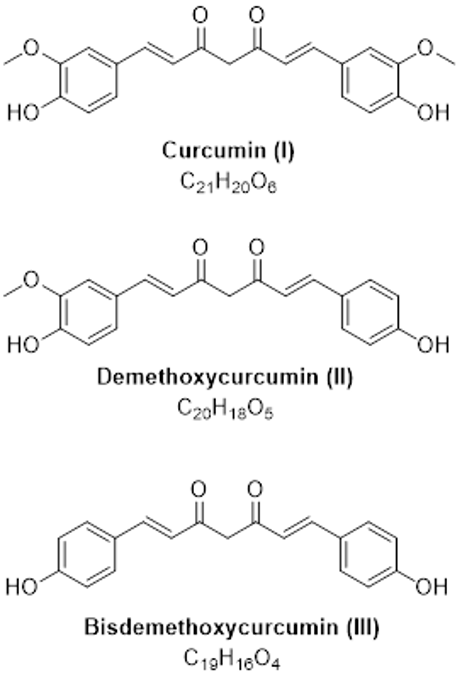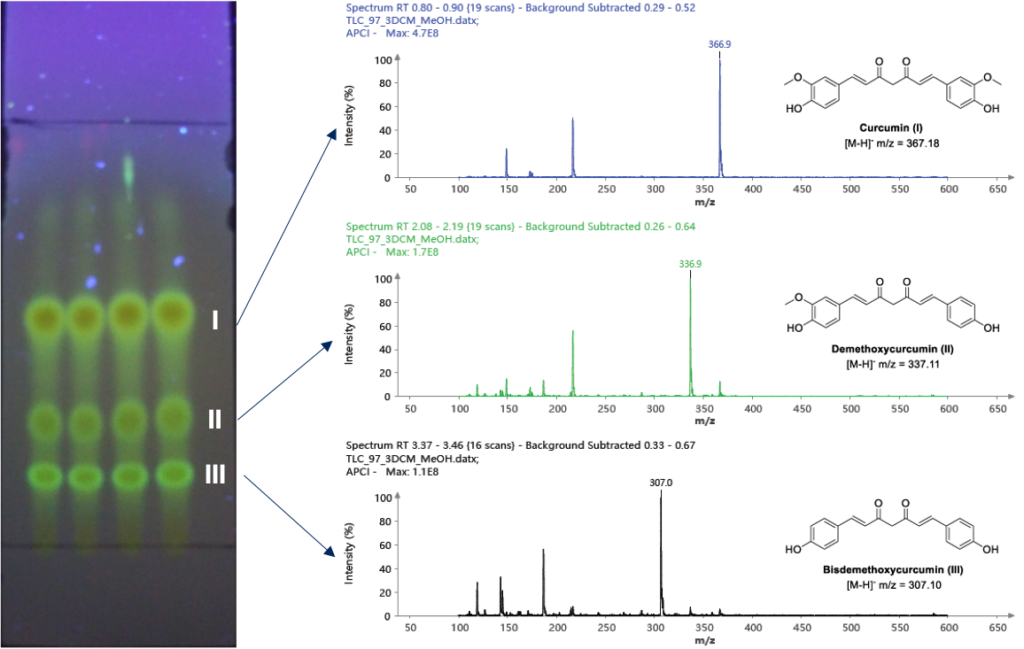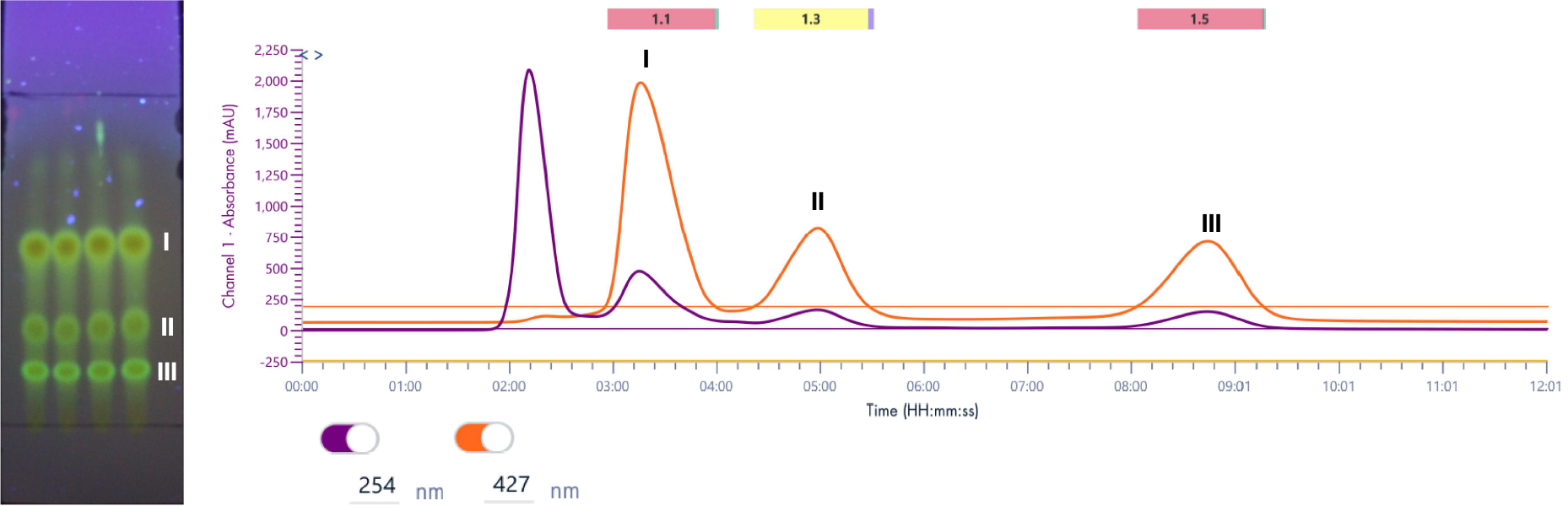
Instrumentation:
Mass Spec: expression® CMS
Sampling: Atmospheric Solids Analysis Probe – ASAP®
Software: MassExpress
Authors
Changtong Hao, Ph.D.
Advion Interchim Scientific
Introduction
In medicinal chemistry, rapid and reliable analytical techniques are crucial for the discovery of new drug products. Mass spectrometry (MS) is a key technique for reaction monitoring that provides essential molecular information about a product at each stage of discovery.
Medicinal chemists often encounter specific challenges when monitoring reactions, such as:
– Ionization Mode Ambiguity: The lack of prior information on the optimal ionization mode (positive or negative) can lead to incomplete data.
– Developing separate methods for each ionization mode may delay progress in a fast-paced field that demands quick insights.
– Limited access to specialized instruments or expertise can also hinder analysis.

Advion Interchim Scientific System
expression® CMS with Atmospheric Solids
Analysis Probe (ASAP®)
Method
The Advion Interchim Scientific® Atmospheric Solids Analysis Probe (ASAP®), integrated with the expression® Compact Mass Spectrometer (CMS) and MassExpress software, uses rapid polarity switching and versatile sample introduction techniques to offering an innovative solution to these challenges:
Rapid Polarity Switching: Seamlessly transitions between positive and negative ion modes, ensuring comprehensive data capture without method changes.
Versatile Sample Introduction: Atmospheric Solids Analysis Probe (ASAP®) enables direct analysis of solid and liquid samples, reducing preparation time.
Enhanced Data Integrity: Accurate identification of compounds with distinct ionization preferences minimizes the risk of missing critical information.
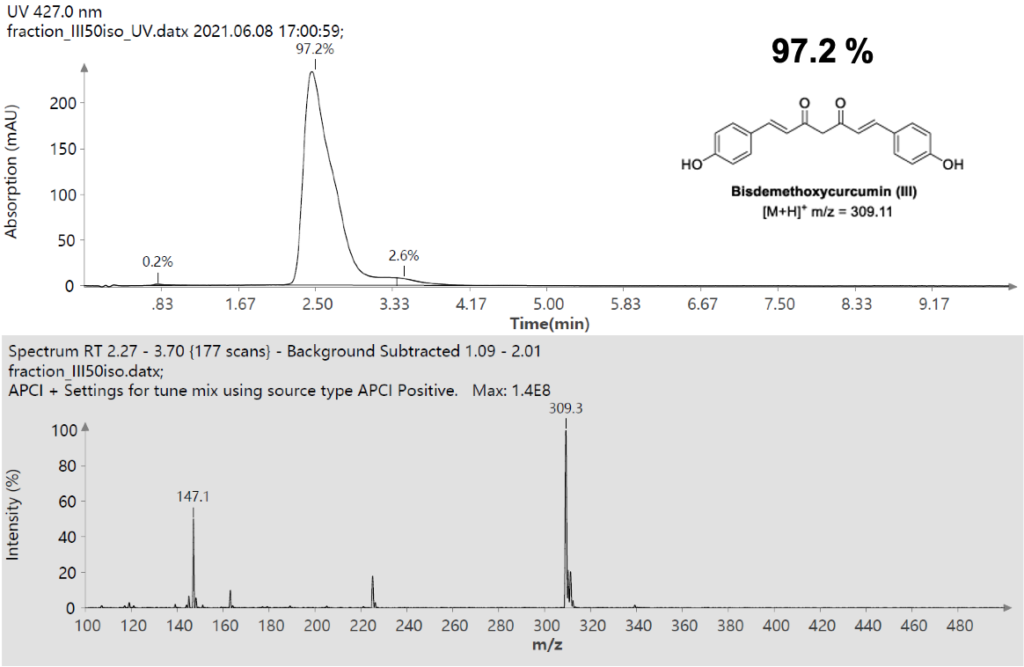
Here is the case where chemist cannot get a successful detection with their traditional LC-MS analysis of the reaction Monitoring with Dual Polarity Detection.
Scenario: Differentiating between a reactant and its product, each exhibiting exclusive ionization behavior.
Reactant (C5H2Br2O2S): Detectable only in negative ion mode, showing peaks at m/z 282.8, 284.8, and 286.8 displaying a characteristic 1:2:1 bromine isotopic pattern.
Product (C6H4Br2O2S): Undetectable in negative ion mode but clearly observed in positive ion mode at m/z 298.8, 300.8, and 302.8, also displaying the same classic 1:2:1 bromine isotopic distribution.

Figure 1: Reaction Path

Figure 2: A top). MS spectrum of starting material in negative mode. B bottom). MS spectrum of product in positive mode.
Using the Advion Interchim Scientific’s ASAP-MS’s rapid polarity switching feature, both compounds were accurately identified within a single analytical workflow and without the need of HPLC separation. Total elapsed time of the analysis was <1 minute for the samples and this technique allows the reaction to be monitored over time.
Conclusion
The Advion Interchim Scientific® ASAP-MS, with its rapid polarity switching and efficient and versatile sample introduction via the Atmospheric Solids Analysis Probe (ASAP®), empowers medicinal chemists to monitor reactions with confidence. The robust system ensures that no critical data is overlooked, streamlining drug discovery workflows and enhancing decision-making processes. Harness the power of dual polarity detection with Advion Interchim Scientific® ASAP-MS—because every detail matters in the mission of drug discovery.