Carbohydrates are non-chromophoric compounds which can often lead to flat signals when using UV detection only. In this application note, we show carbohydrate purification with ELSD and how such detection can help in purification.
The ELSD detector that AIS proposes is capable of providing several benefits to the user, including:
- No need to define gain depending on the loading.
- The ability to see more compounds (especially temperature sensitive or volatile) thanks to low temperature technology.
- Removal of water response – In this application we used water and 35°C was enough to remove water response and get a flat signal.
In this application note, we compare the use of a flash chromatography system with a UV detector alone versus the same system paired with a mass spectrometer. The data highlights the impact on workflow efficiency and underscores the importance of selecting the appropriate splitter.
The specificity of MS detection improves the efficiency of the purification workflow (compared to traditional Flash and HPLC).
With any growing lab, scientists find an increasing list of needs to improve in throughput, capabilities, or just the addition of staff to your team. This can result in several outcomes that make it difficult to meet environmental and sustainability goals, or a laboratory’s bottom line.
By building flexibility in your lab with multi-use instrumentation, scientists can help improve some of the biggest pain points in the purification process, including run-times and solvent use, leading to an overall lower cost of ownership.

Q: What is the focus of your lab’s research?
A: My work focuses on the synthesis and purification of carbon nano assemblies that can be used in agricultural applications. These materials, which include graphene quantum dots (GQDs), carbon quantum dots (CQDs), carbon nanodots (CNDs), and polymer dots (CPDs). These nanomaterials can utilize wavelengths from the solar spectrum that are not usually accessible to plants, thereby transferring electrons to the light harvesting complex via fluorescence or direct electron transport. Due to their organic composition the carbon nano assemblies are biodegradable, providing a safe removal route post application. In the future, optimizing bottom-up synthesis approaches for carbon nano assemblies can improve their quantum yields, making them a greener alternative to greenhouse films based on inorganic quantum dots.
Q: Why did you incorporate the puriFlash® into your laboratory?
A: In our lab we use the puriFlash® 5.125 model. The automated system ensures continuous operation without manual intervention, which frees up a lot of time in the lab. The instrument also offers precise control over parameters like flow rates, gradients, and pressure, enhancing reproducibility. Additionally, the system provides continuous monitoring of key parameters (Absorbance above threshold, retention time) and real-time adjustments to optimize performance can be done quickly, due to the flexible programming.
Who would you recommend purchasing the puriFlash®?
A: I think a system like the puriFlash® 5.125 should be incorporated into most synthetic labs. The pump on this instrument is very powerful, and can reach pressures of 125bar, whilst permitting liquid and solid loading to the columns. These systems also permit modifications, as the UV detection can be extended from 200-400nm all the way to the 600nm range.
Q: Do you have any publications or presentations using the puriFlash®?
A: Below I provide a few graphs from some unpublished data. I used a puriFlash® PF-3-0C18HP column with a 2ml loop injection, an MeOH: H2O mobile phase (flow rate 15ml/min), using a 95:5 = > 5:95 gradient. 10 different carbon nano assemblies were separated from the reaction mixture that displayed different absorbance and emission spectra.
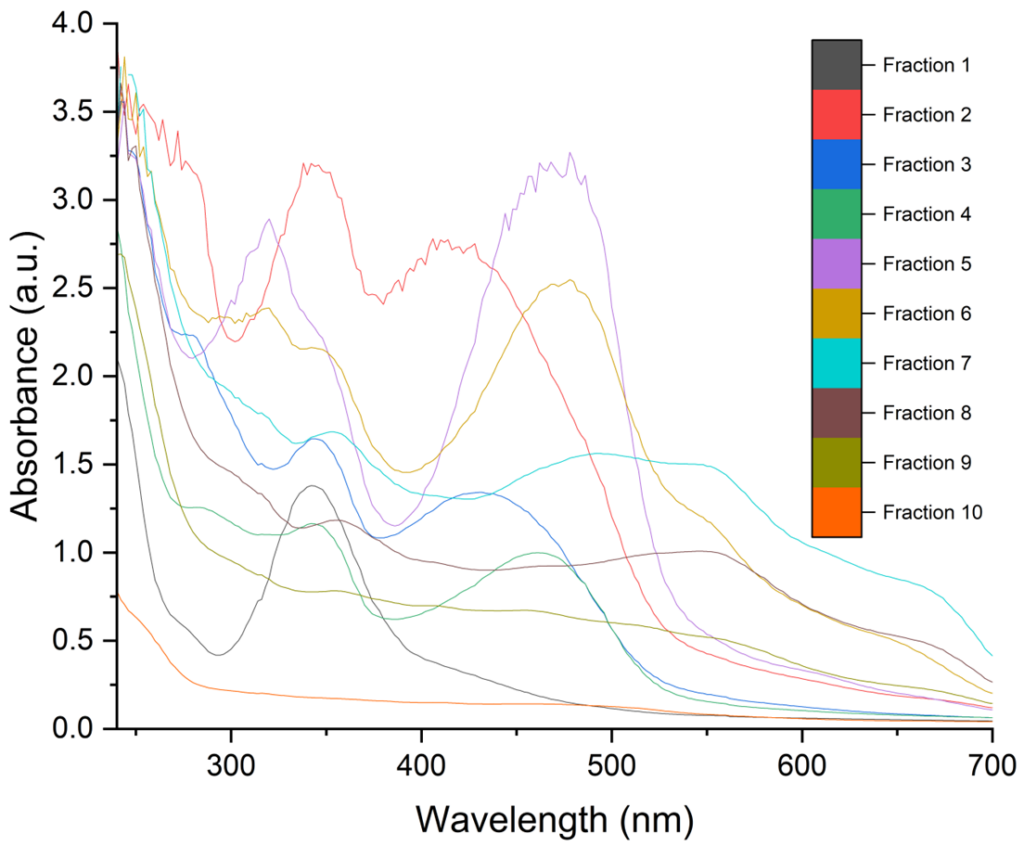
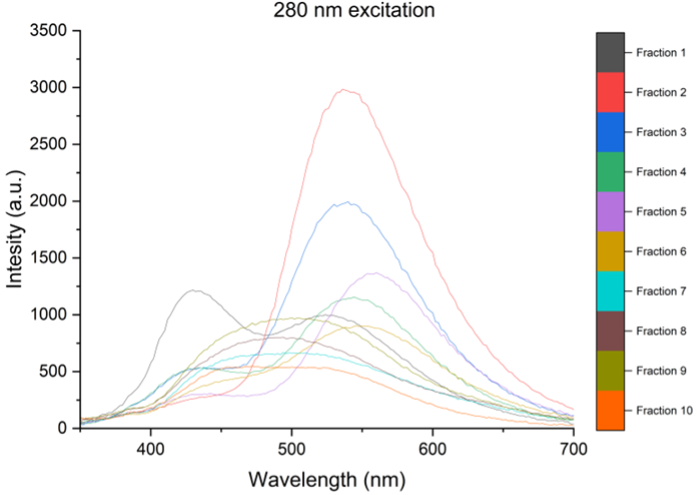
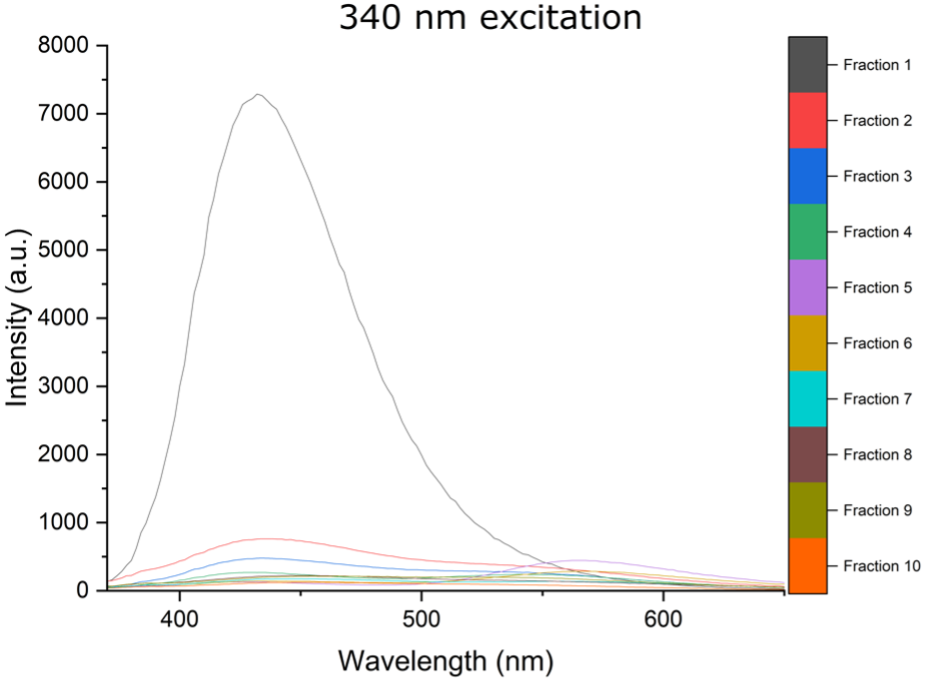
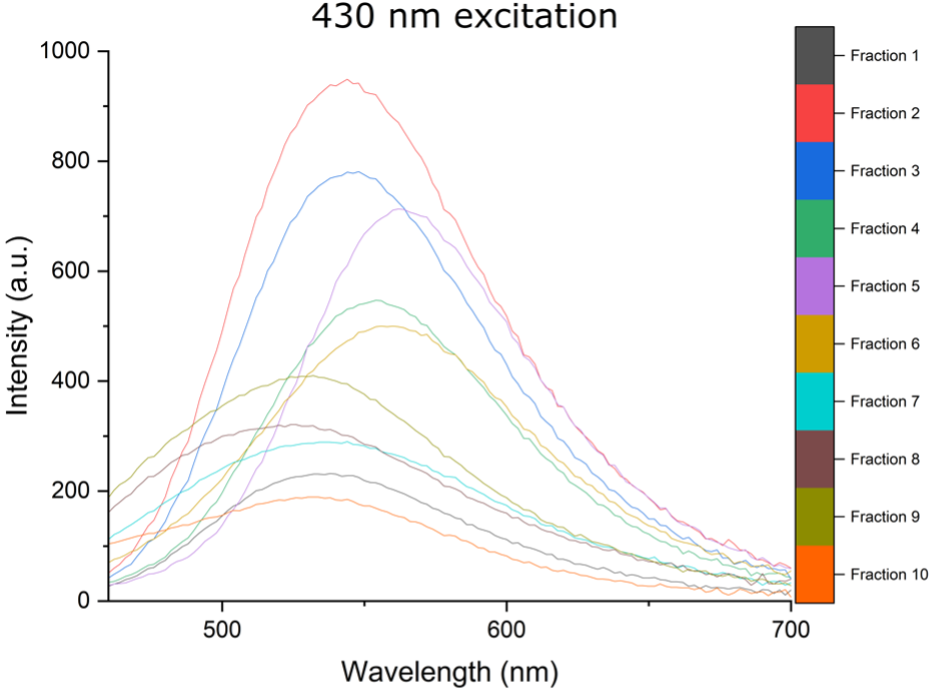
We propose that these supramolecular carbon nano assemblies are held in place π-π stacking and hydrogen bonds.
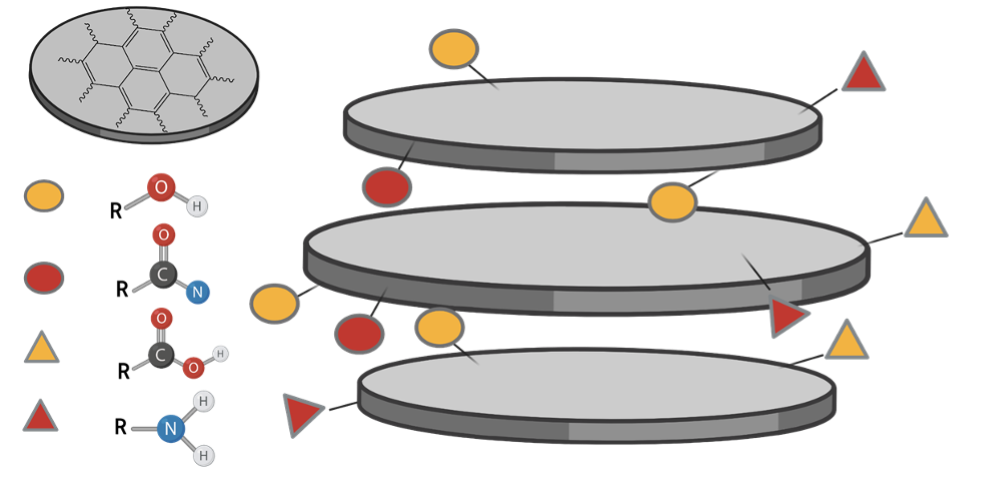
The fluorescent fractions are then used in the plant photosystems to increase the photosynthetically active radiation, by absorption of UV radiation and conversion to blue, green and red light.
Genius Method Development Software: Your daily assistant for automated method transfer
Recorded Thursday, October 24, 2024
With any system in the puriFlash® range, you will benefit from Genius, the artificial intelligence of the InterSoft® X software suggesting the best purification method for you based on your TLC and HPLC data. No matter how skillful you are with chromatography, Genius will enable you to increase the productivity of your laboratory and to achieve expert results for all your purifications.
In this webinar you will learn:
– How Genius can provide automatic detection of compounds.
– Easy and automatic calculation of Rf and ΔCV.
– Direct (and secure) transmission of this information to the puriFlash® system. “Genius”, integrated into the software, will suggest the best method for successful purification.
– How to easily archive your TLC data.
Speaker: Romain Spadaro
Flash Purification Product Manager
Advion Interchim Scientific®
This Webinar was originally presented November 9, 2023 on LabRoots.com
ABSTRACT
In this webinar, you’ll learn how to address your lab’s purification pain-points with methods and instrumentation to maximize your budget, time and increase overall efficiency. During this session you will discover:
- How to select the ideal purification solution for your lab’s specific needs
- How to optimize your purification workflow daily, to automate compound ID and fraction confirmation – using simple, prep free tools
- How your cell phone can be used for automated method development
- How column selection factors in to your workflow, and why exploring what is best for your method matters
SPEAKER
Chase Needham, puriFlash and PrepLC Applications Scientist, Advion Interchim Scientific®
In this application note, we use whey protein isolate and two casein macro peptides as an example to demonstrate the isolation and purification of bioactive peptide components with a system comprised of a puriFlash® 5.250 prepLC coupled online to an expression® CMS detector.
In this application note, we demonstrate a complete solution for the synthesis, purification, and quantitation of Gd(III)-DOTA, an ionic imaging contrast agent with better chemical stability and lower toxicity compared to the currently used Gd-(DTPA).
In this application note, we show how Cyanidin-3-glucoside can be extracted from black rice and purified with a prepLC/Flash system. The amount and purity of Cyanidin-3-glucoside was measured utilizing a certified reference standard.
In this application note, a method to separate and purify green tea catechins with preparative liquid chromatography (Prep-LC) using the puriFlash® 5.250 system is demonstrated. An additional HPLC-UV-MS method is also demonstrated for compound confirmation and purity analysis.






