
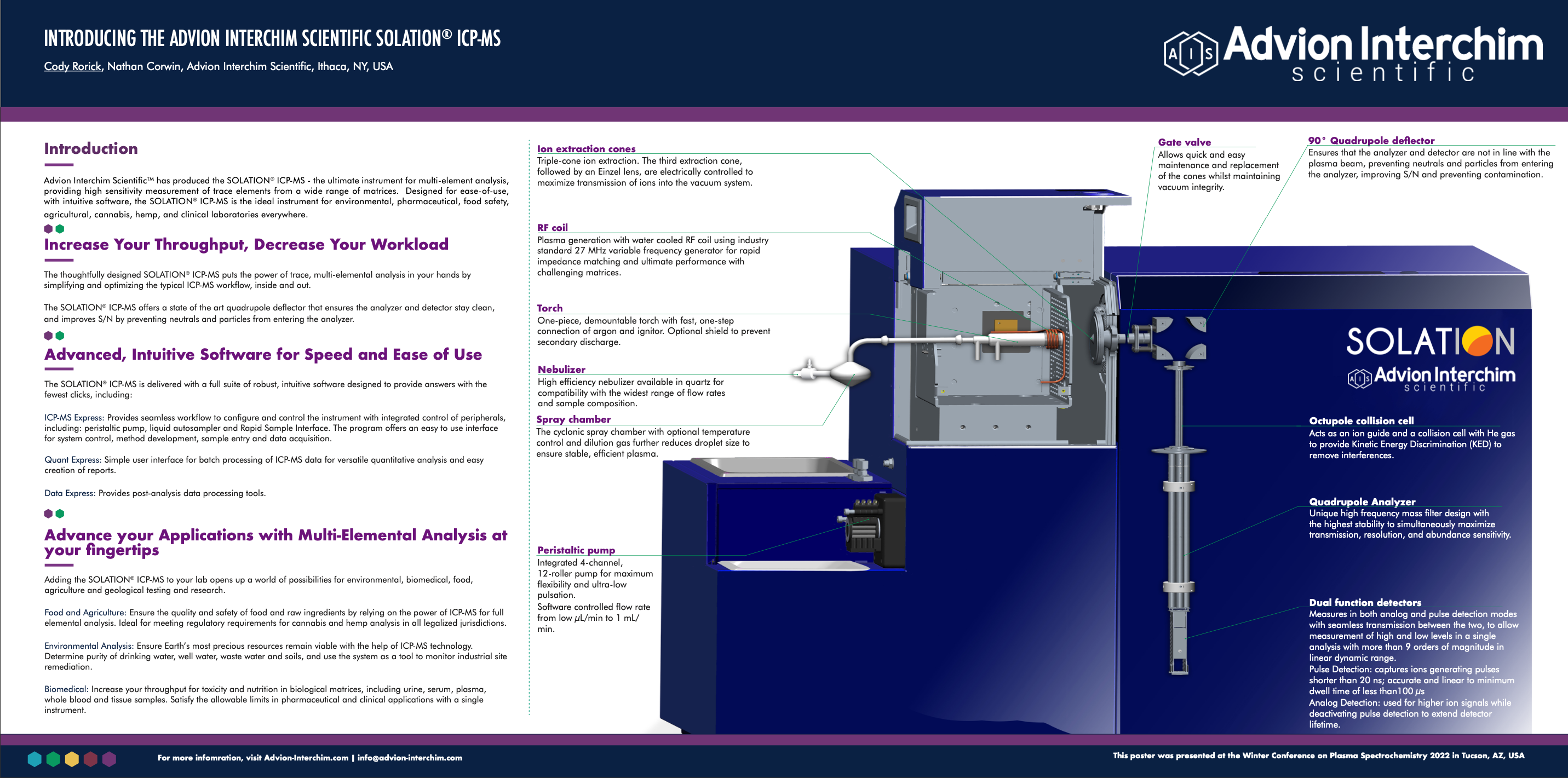
Instrumentation: SOLATION® ICP-MS
INTRODUCTION
With the growing acceptance and legalization of hemp and cannabis in the US, Canada, and several other countries, cannabis products are more widely available than ever before. Now approved for medical, recreational and health supplement uses, increased production and consumption have highlighted the need for routine testing and development of testing standards for toxic chemicals, including heavy metals, in cannabis plant material, and all the byproducts made from them, to ensure safe products for the consumer. With the adoption of chapters <232> and <233>, US Pharmacopeia (USP) specifies a list of elements and maximum exposure limits based on toxicity and routes of administration for pharmaceutical products.
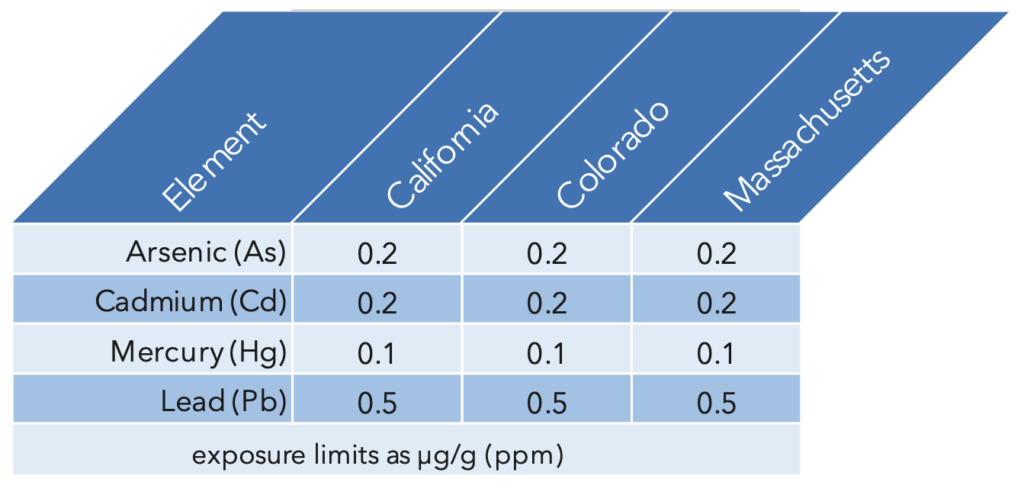
Many states that have legalized medical and recreational cannabis base their exposure limits on the USP values. California, Colorado, and Massachusetts are examples with Permissible Daily Exposure (PDE) limits by inhalation for As, Cd, Hg, and Pb. These values are summarized in Table 1.
Table 1: PDE limits for states that use the USP guidelines for heavy metal exposure by inhalation. USP<233> also defines the accuracy, repeatability, and ruggedness required for the analysis of these toxic elements:
![]() Validation Criteria
Validation Criteria
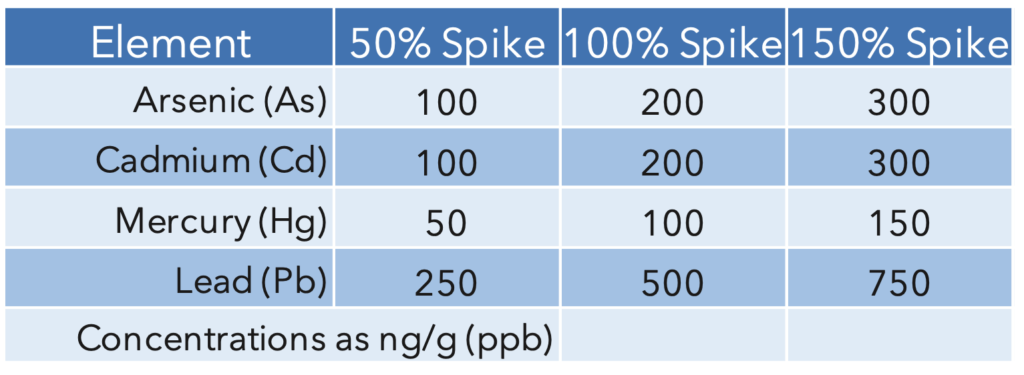
Accuracy: The matrix and materials under investigation must be spiked with target elements at concentrations that are 50%, 100%, and 150% of the maximum permitted daily exposure (PDE). Mean spike recoveries for each target element must be within 70%-150% of the actual.
Repeatability: Six independent samples of the material under investigation must be spiked at 100% of the target limits defined and analyzed. The measured percent relative standard deviation (%RSD) must not exceed 20% for each target element.
Ruggedness: Carrying out the repeatability measurement testing procedure by analyzing the six repeatability test solutions either on different days, either with a different instrument or by a different analyst. The %RSD of the 12 replicates must be less than 25% for each target element.
In this study, we used the Advion SOLATION® ICP-MS and a microwave digestion system to digest and analyze hemp samples using the validation methods described in USP General Chapter <233>. The sensitivity, ability to handle complex matrices, and the ability to remove interferences with a helium collision cell makes this the ideal system for heavy metal analysis in the cannabis industry.
EXPERIMENT
Sample Preparation
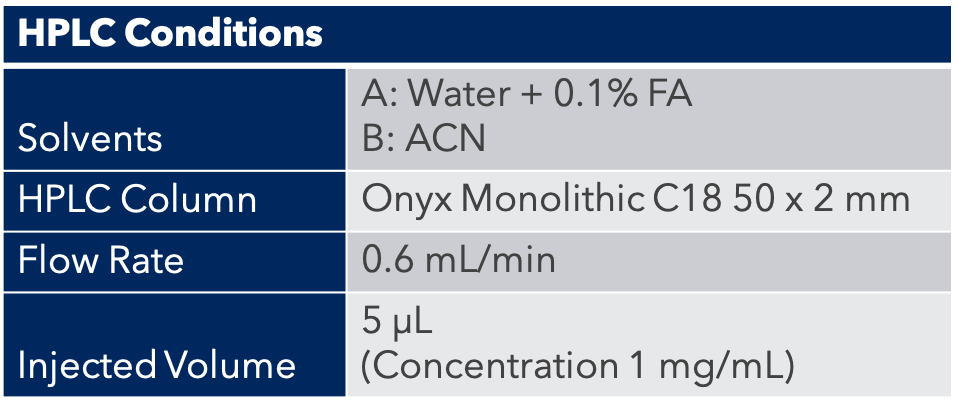
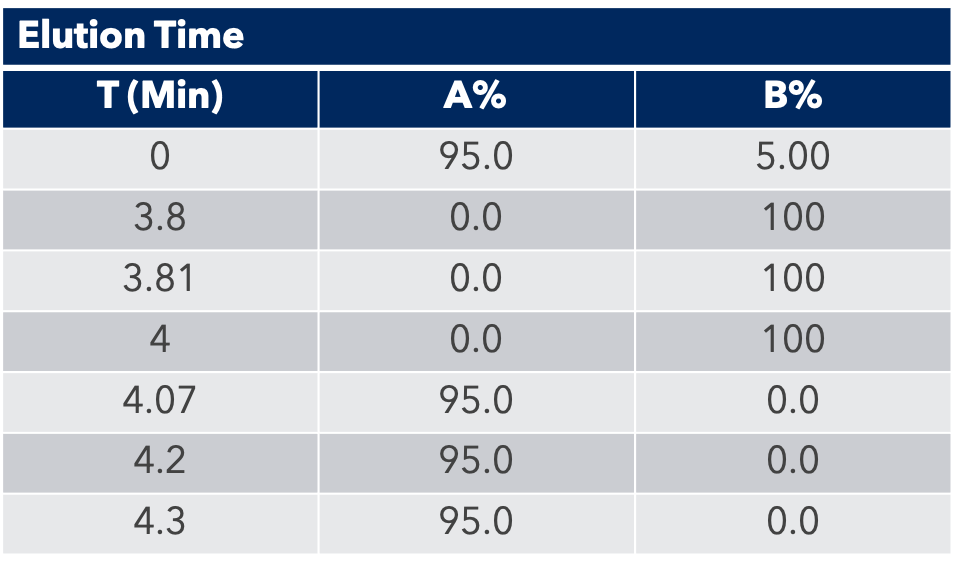
A sample of medicinal hemp flower was purchased locally. Around 14 grams was finely ground and homogenized, then 0.5g +/- 0.005g was weighed into digestion vessels and the appropriate amount of a spike solution added. Nine mL of concentrated HNO3 and 1 mL concentrated HCl was added to each vessel and the samples were allowed to react for 15 minutes prior to sealing and placing the vessels on the turntable of the closed vessel microwave digestion system. The program controls the microwave energy such that the samples ramped up to the optimal digestion temperature of 200°C over 20 minutes, maintained 200°C for 10 minutes, and then cooled back to room temperature.
This method resulted in the complete digestion of all samples resulting in a clear, particulate-free solution once brought to volume with 18MΩ water in a 50mL volumetric flask.
The calibration standards and spikes were based on the action levels in table 1. The sample set included a hemp sample, a duplicate, the 50%, 100%, and 150% spikes, and NIST 1575 Pine needles to further validate the results, which were run in duplicate. For the ‘ruggedness’ specification from USP<233>, there were 6 samples of the 100% spiked hemp. The spike values are summarized in Table 2.
Table 2: Spike values based on the action limits defined in USP<233>
![]()
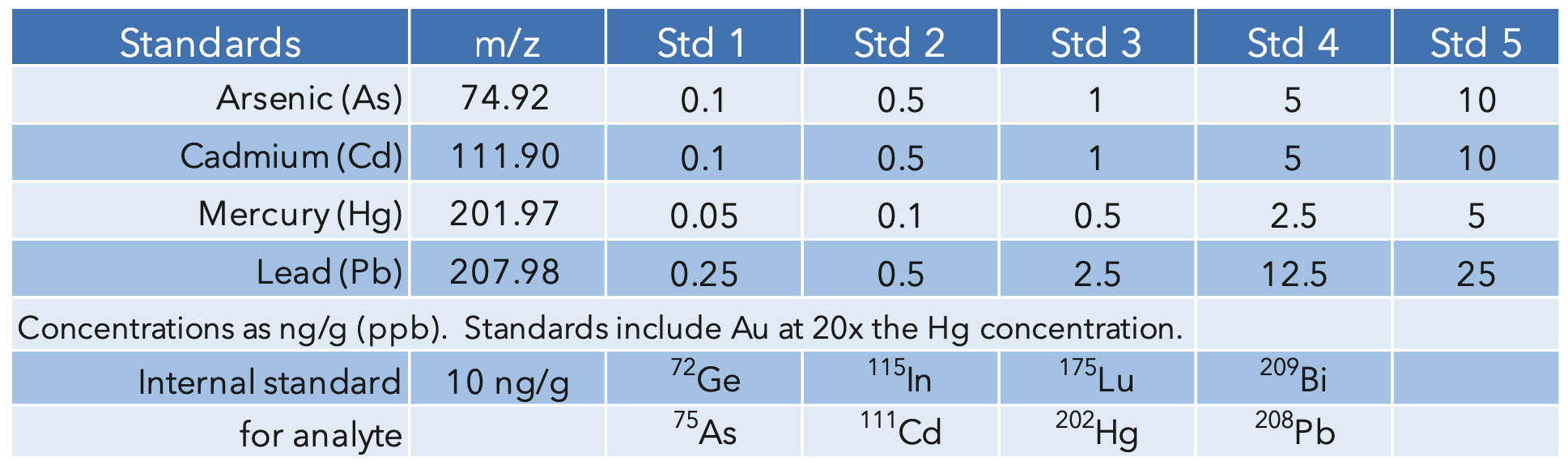
A second, 1:4 dilution was performed post digestion to bring the final acid concentration to 5% for an overall dilution factor of 400x. The calibration blank and standards were prepared using the same acid concentration, 5% of 9:1 HNO3/HCl, for matrix matching. To stabilize mercury and help with wash-out, Gold was added at 20x the mercury concentration. The internal standards were added to all the samples, standards and blank for a final concentration of 10 ng/g (ppb). The standard concentrations and internal standards are summarized in Table 3.
Table 3: Analyte masses, calibration standards, and internal standards.
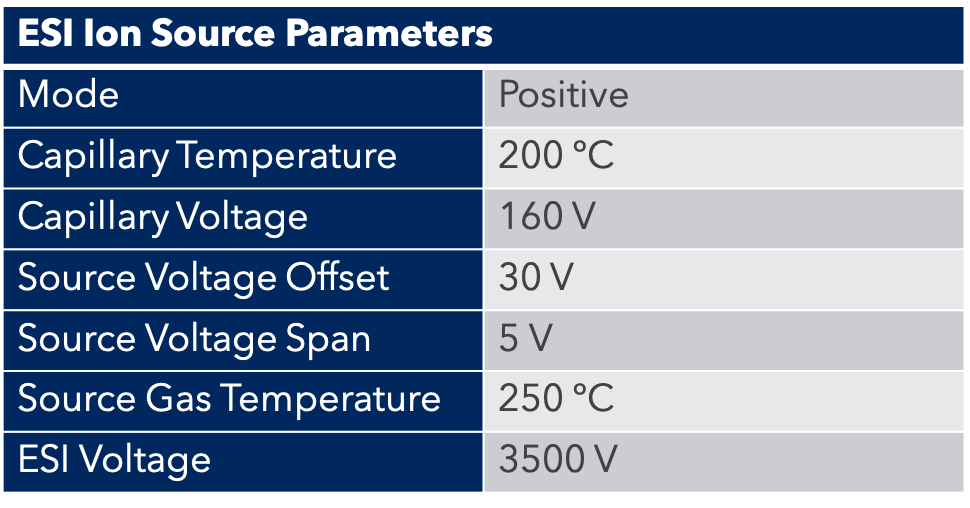
Instrumentation
The Advion SOLATION® ICP-MS incorporates a robust solid-state generator, orthogonal ion optics for keeping the most sensitive components of MS clean, and easy-to-use control and data processing software.
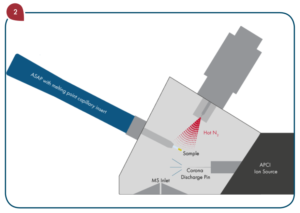
Since HCl was used in the sample digestion, there is a significant amount of chlorine present which creates an isobaric interference on 75As from 40Ar35Cl+. The collision cell effectively eliminates the contribution that ArCl+ makes to the signal at m/z 75 by taking advantage of kinetic energy discrimination (KED) to separate polyatomic interferences from analyte ions resulting in accurate quantitation of low levels of Arsenic. Arsenic is the only analyte in the suite with this type of interference, so the collision cell isn’t used for Cd, Hg, or Pb.
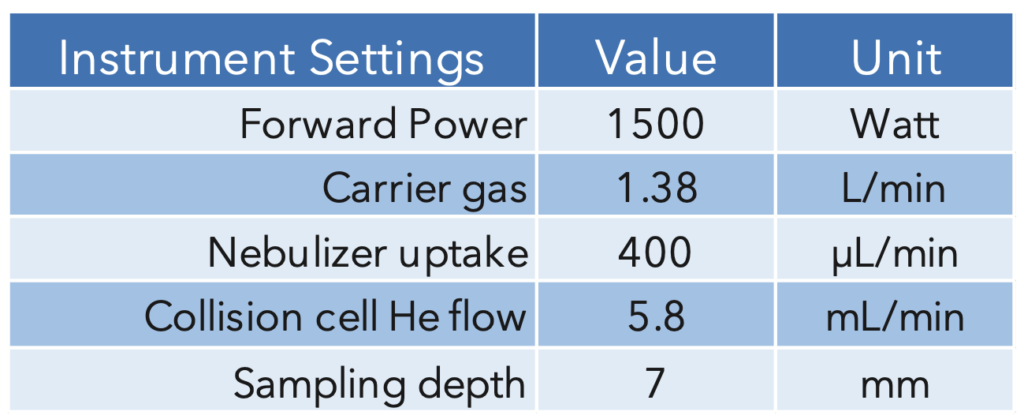
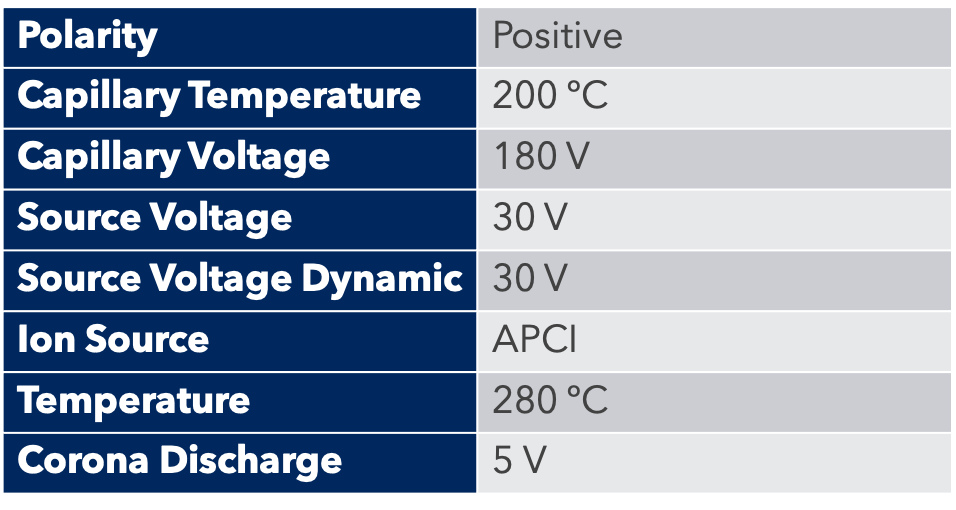
A glass concentric nebulizer fitted to a cyclonic spray chamber, connected to the standard torch with an injector ID of 2mm, was used for sample introduction. Instrument running parameters are summarized in Table 4.
Table 4: ICP-MS Parameters
RESULTS AND VALIDATION
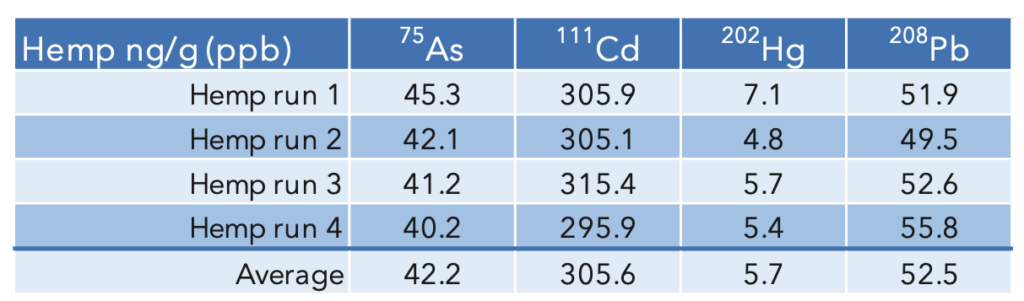
Sample Results![]()
The concentrations of mercury and lead in hemp were less than the lowest standard and all values were less than the action limits. The samples were prepared and analyzed in duplicate, and the average of those duplicates is shown in Table 5. In keeping with the ruggedness requirement, the samples were run on separate days by two different analysts.
Table 5: Hemp sample results (average of the sample and duplicate)
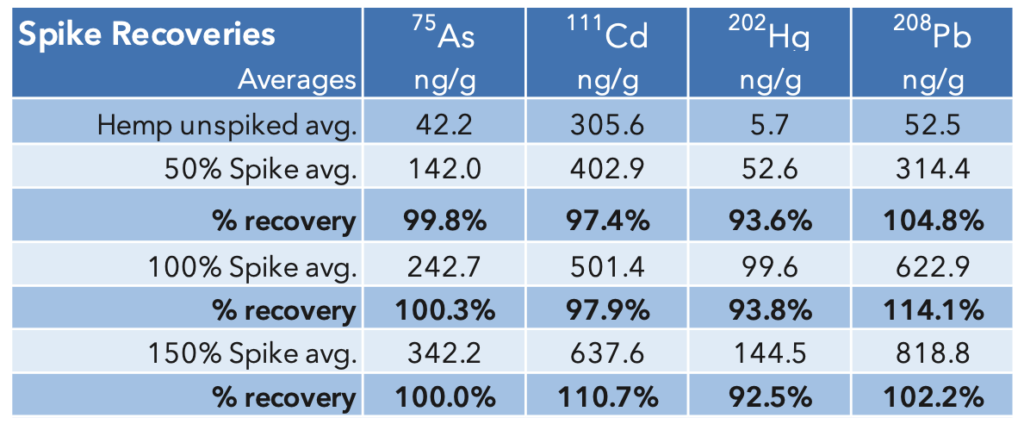
Accuracy: The samples were spiked at 50%, 100%, and
150% of the action level (Table 2 above) and the percent recoveries calculated. Spike recoveries were all between 92.5% – 114.1%, well within the 70-150% range defined by the USP method.
Table 6: Accuracy- Spike recoveries
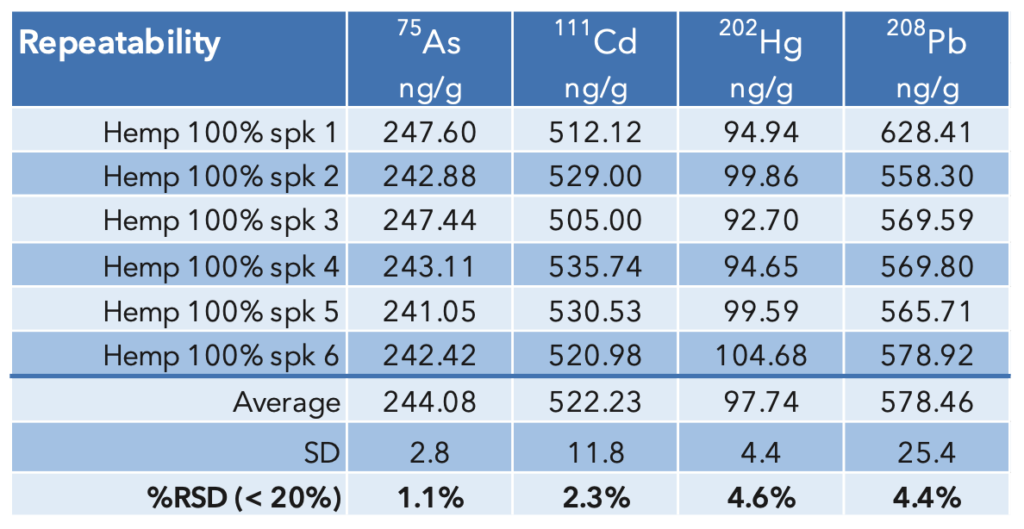
Repeatability: Six hemp samples were spiked at 100% of the action level and digested. The results that are summarized in Table 7 show that the %RSD of the measured concentrations are between 1.3% – 3.7%, demonstrating repeatability well below the 20% limit.
Table 7: USP<233> Repeatability results
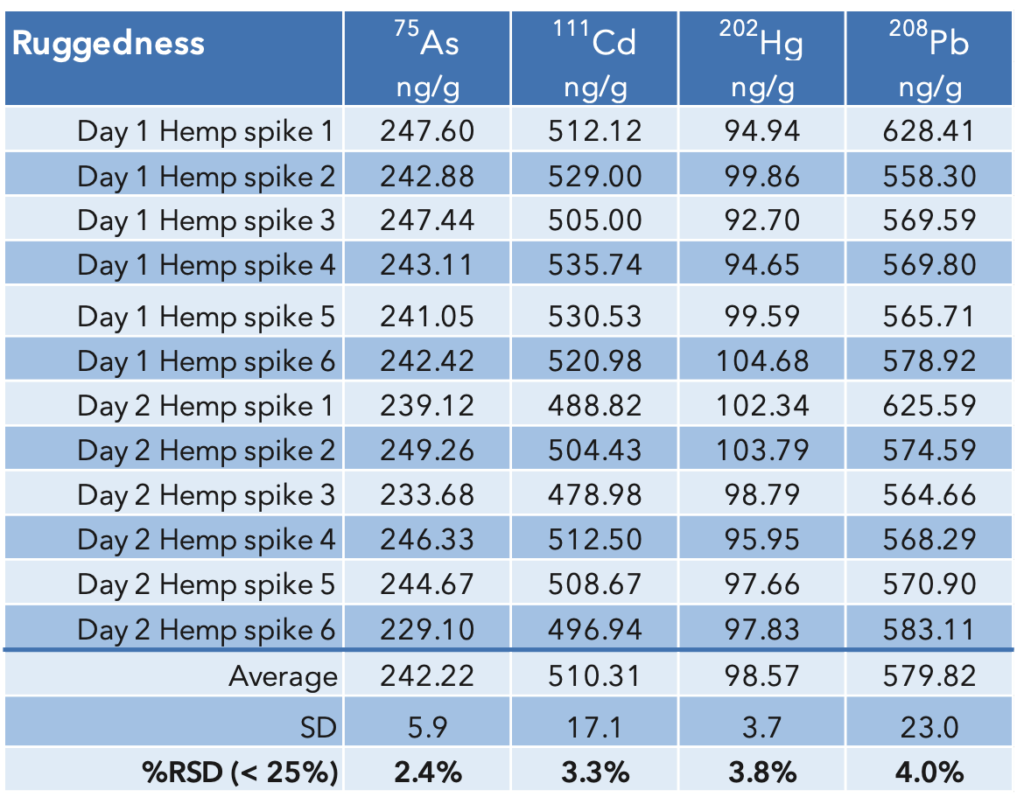
Ruggedness: The repeatability sample set was prepared and run on a different day by a different analyst. The results from that run are combined with the previous run to determine the ruggedness. The ruggedness values are similar to the repeatability values and the measured %RSD (2.4 – 4.0%) are comfortably under the 25% limit defined by the USP method. The results are summarized in Table 8.
Table 8: USP<233> Ruggedness results
NIST 1575a Results
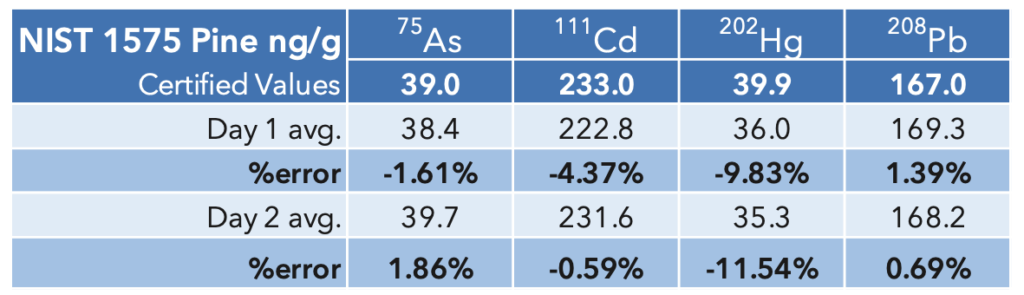
The results for the NIST SRM are summarized in Table 9. The values for As and Hg were less than
the low standard in solution but there is good agreement between the experimental values and the certified values.
Table 9: NIST 1575a Pine Needles SRM
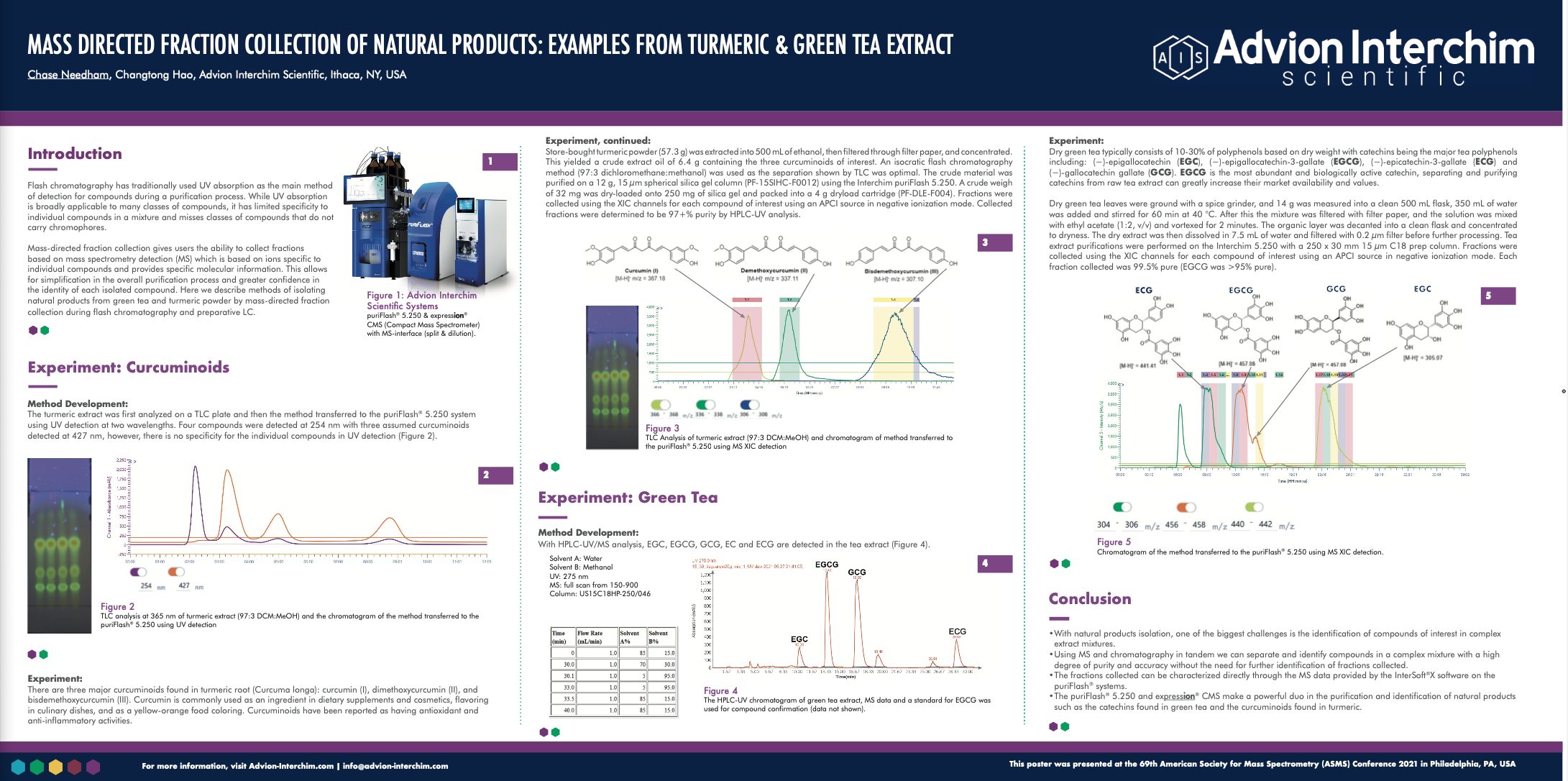
CONCLUSION
This study demonstrates that the Advion SOLATION® ICP-MS , coupled with a microwave digestion system, is suitable for the accurate, robust and reproducible analysis of heavy metals in hemp plant material – greatly exceeding the requirements of USP <233> protocol.
Validation of the microwave digestion method was reinforced by the excellent recovery results obtained for the NIST SRM 1575 Pine.













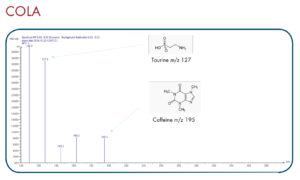
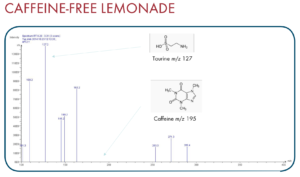

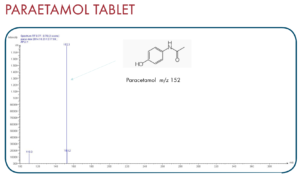



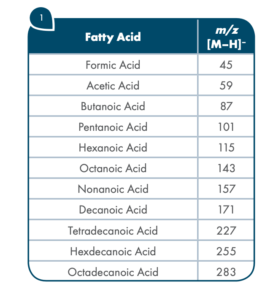





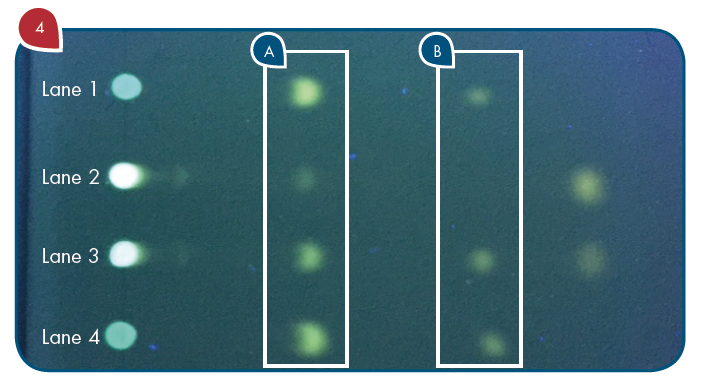
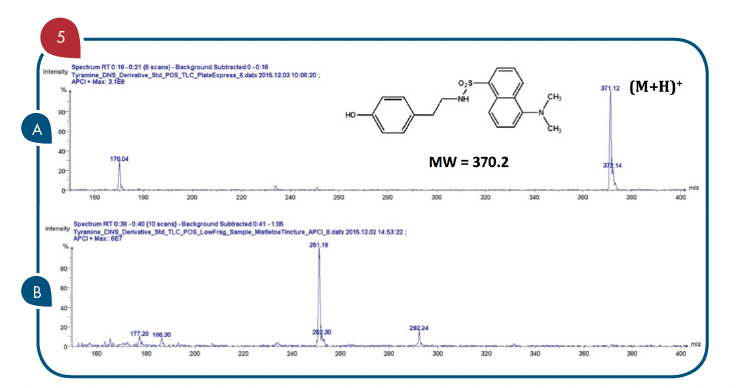

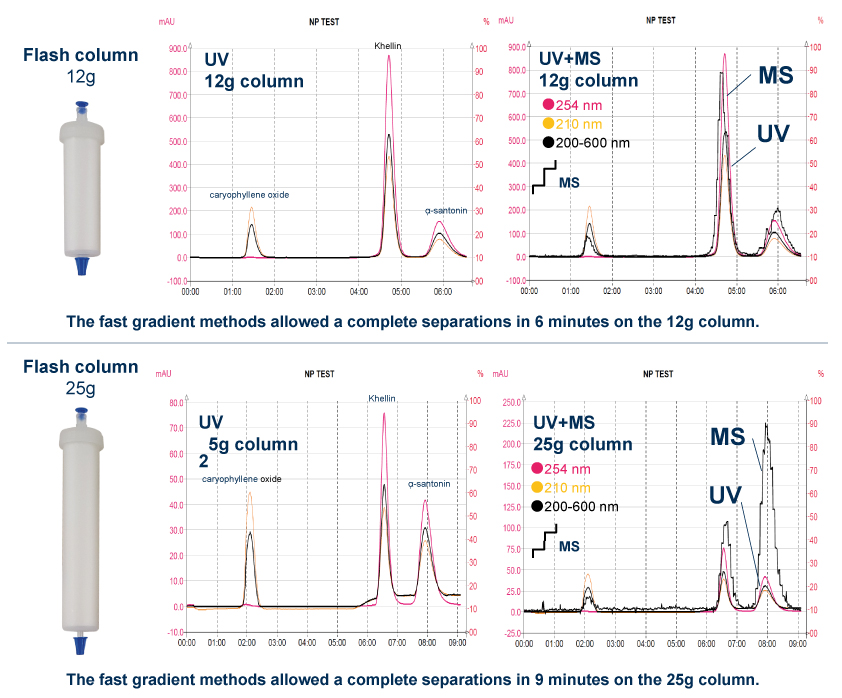
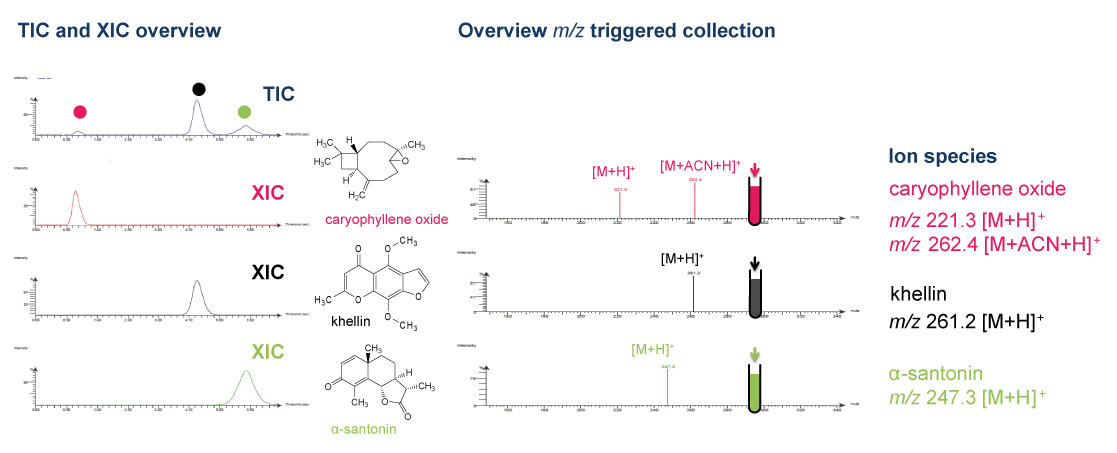
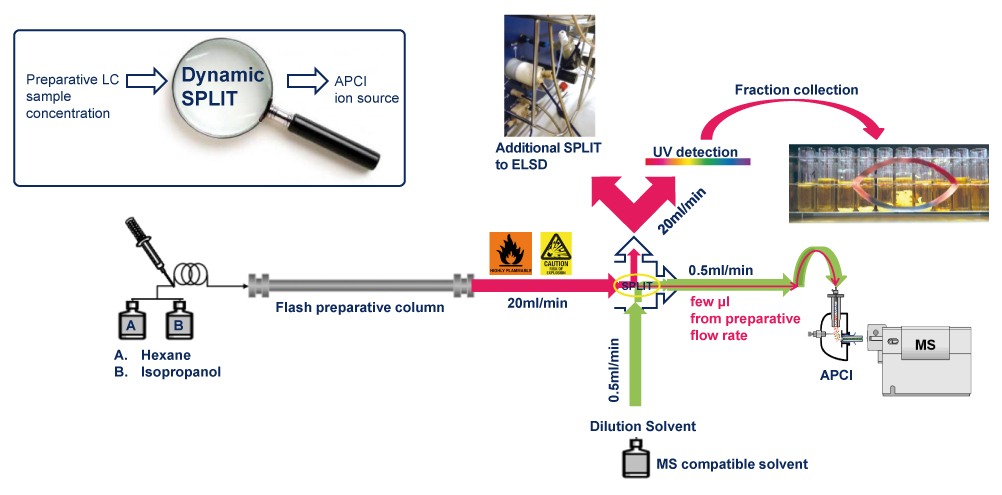
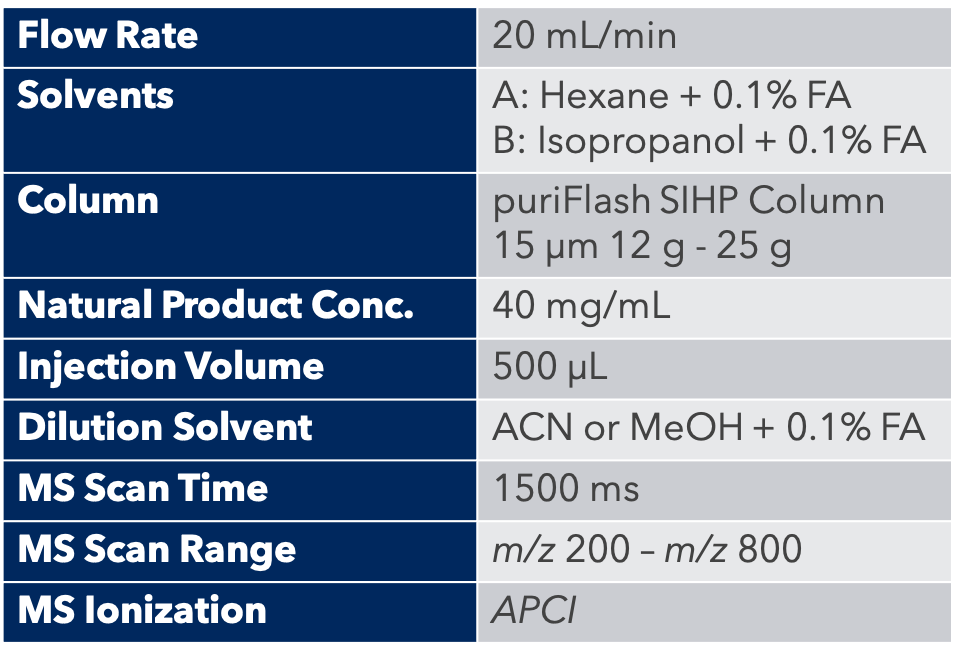
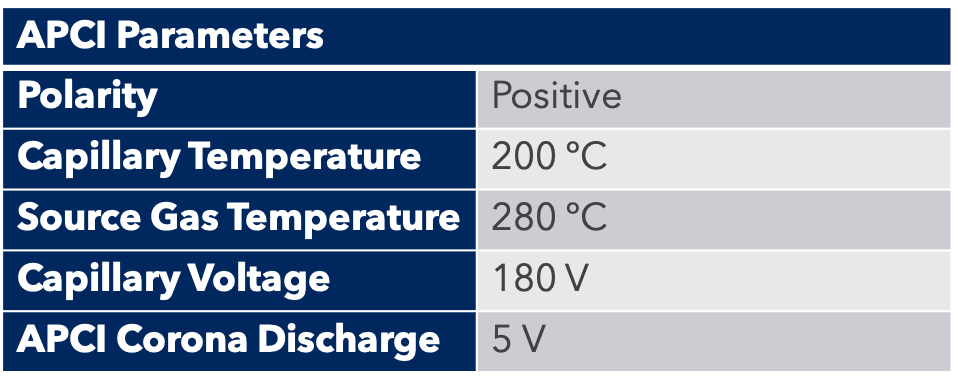



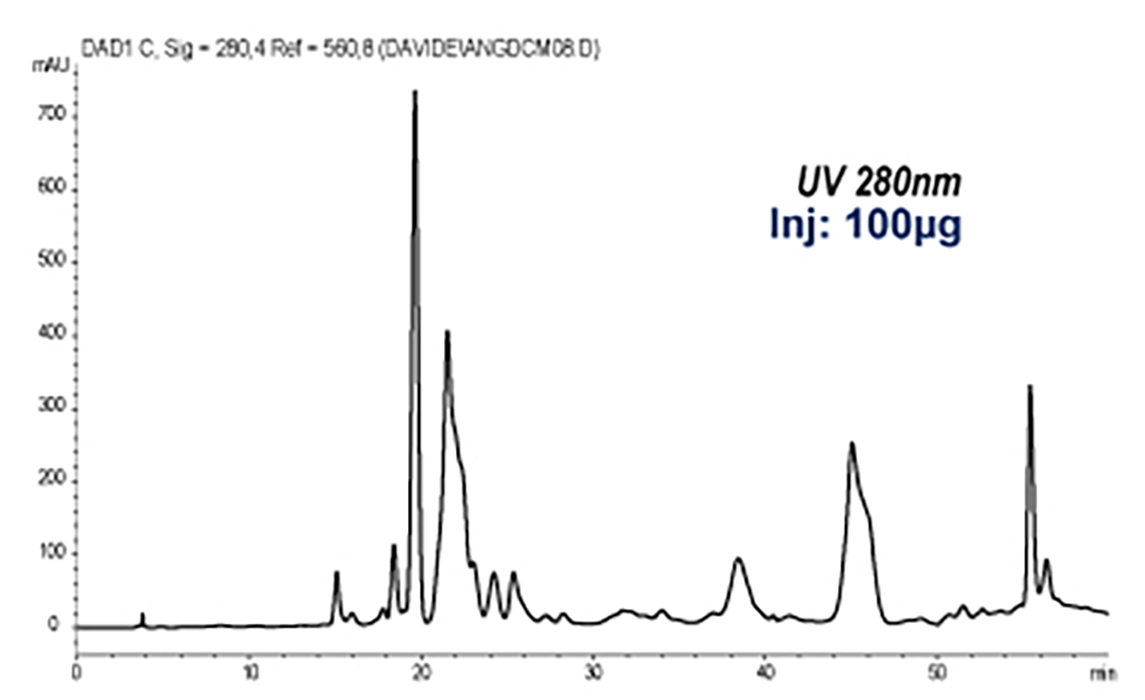
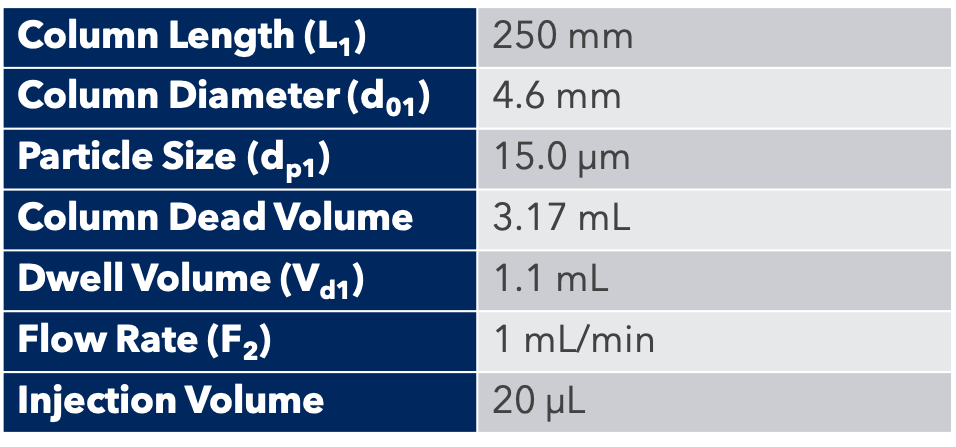
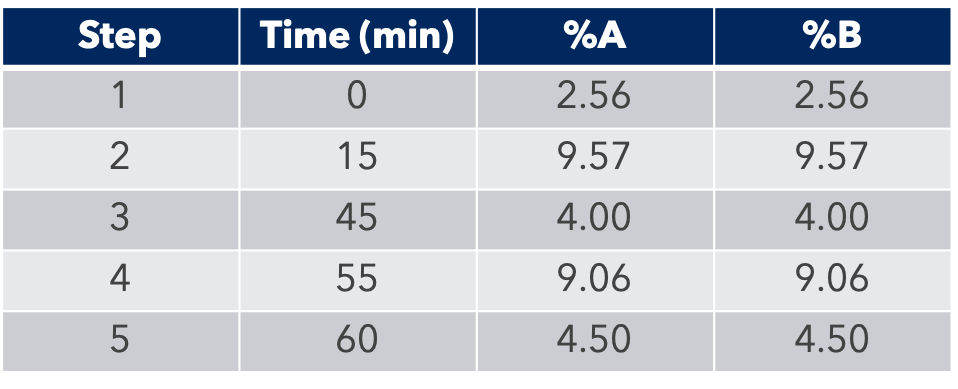
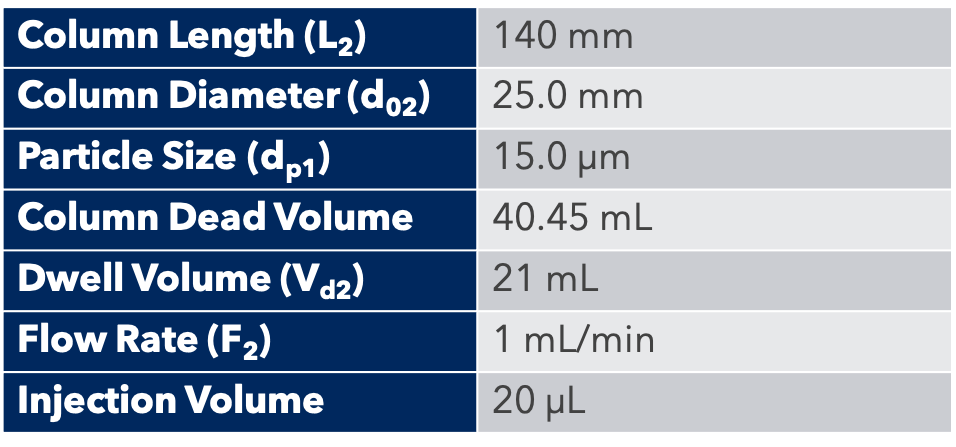

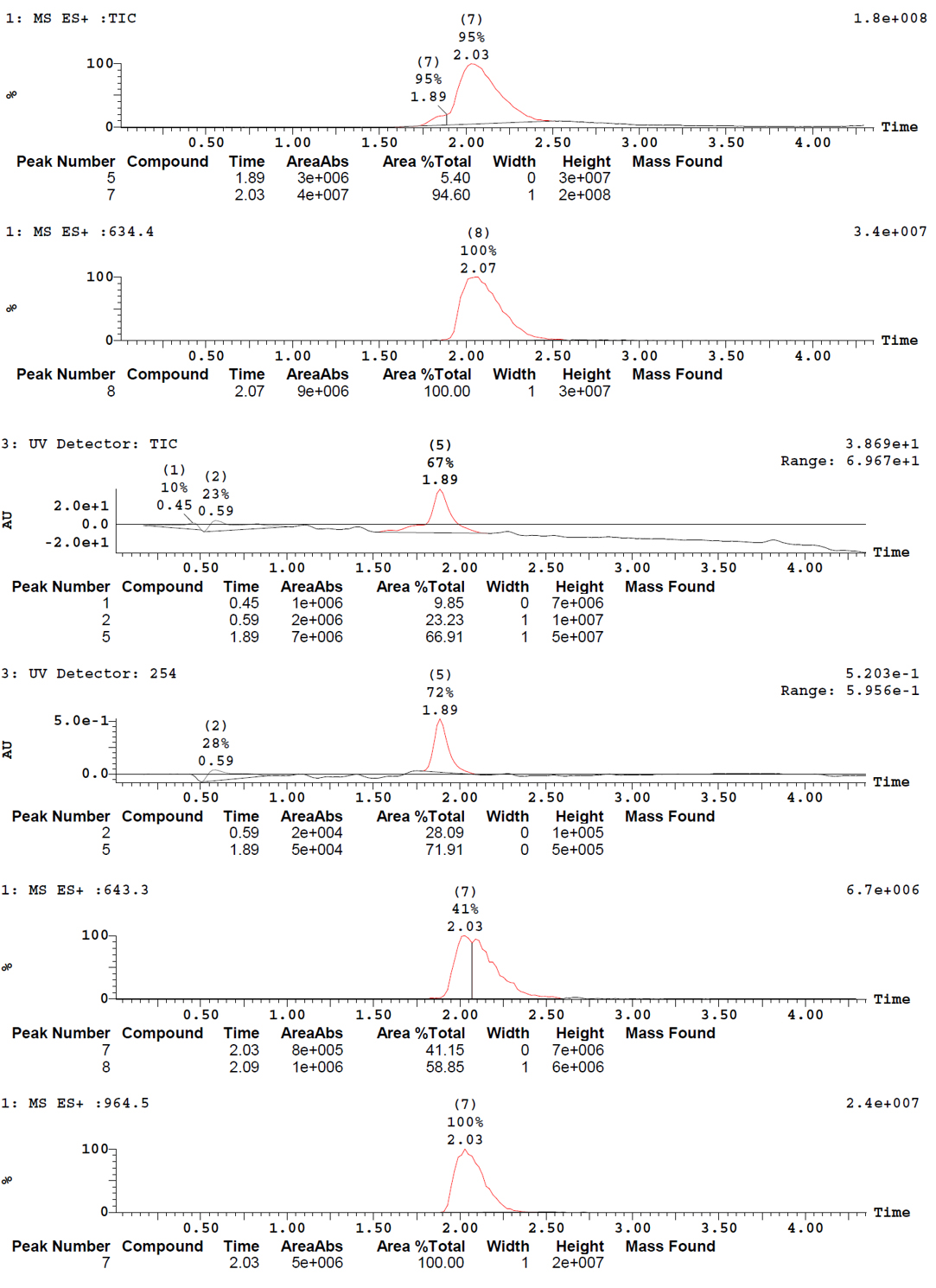
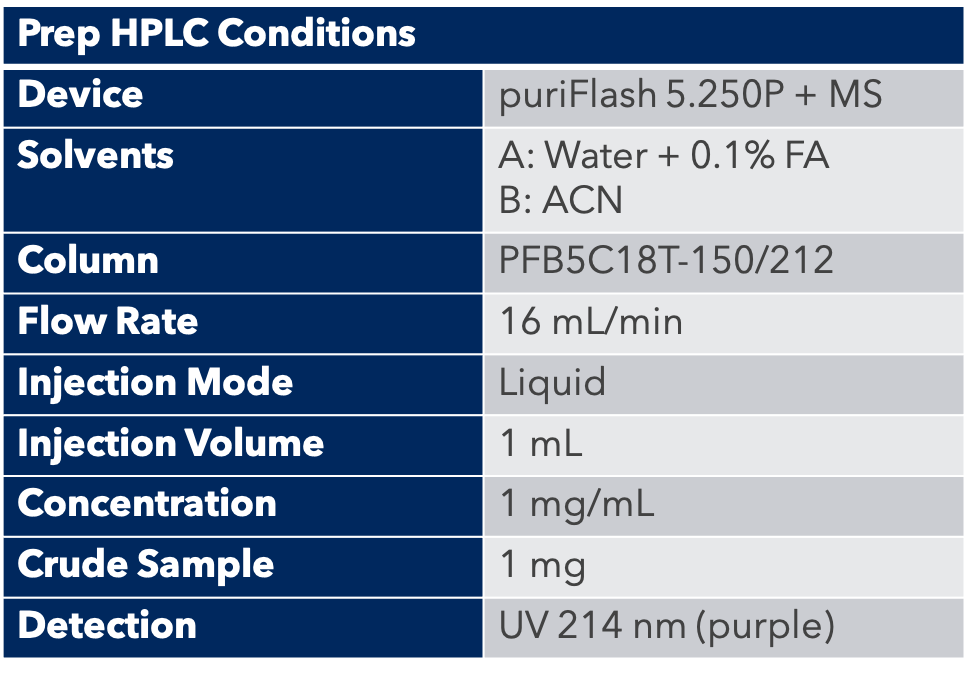
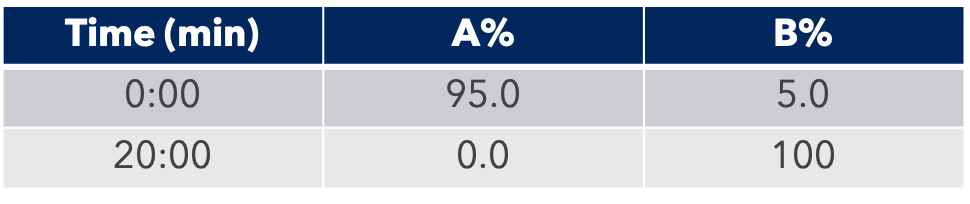
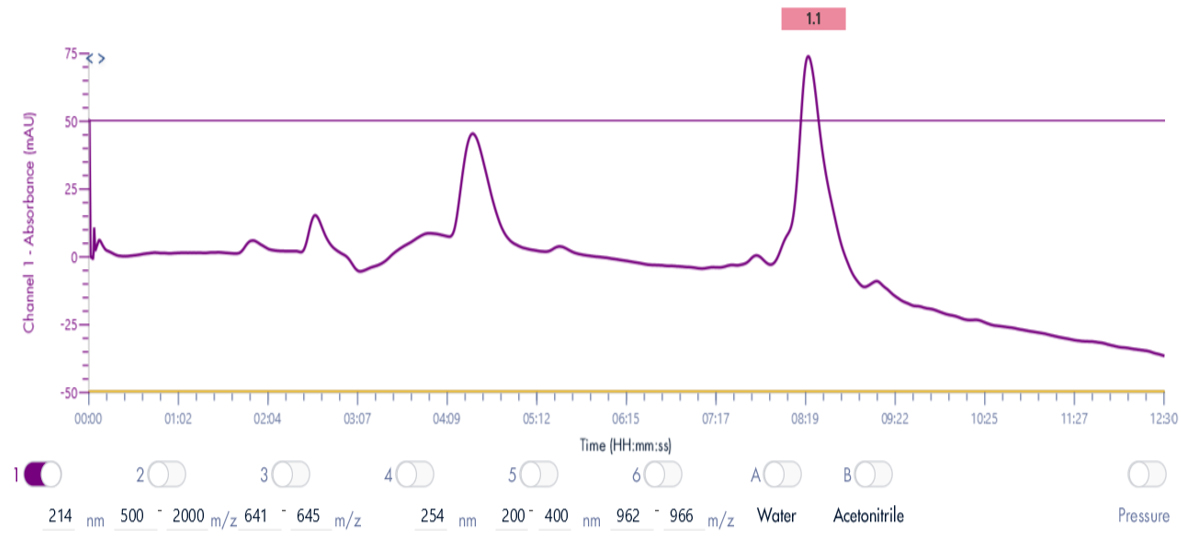
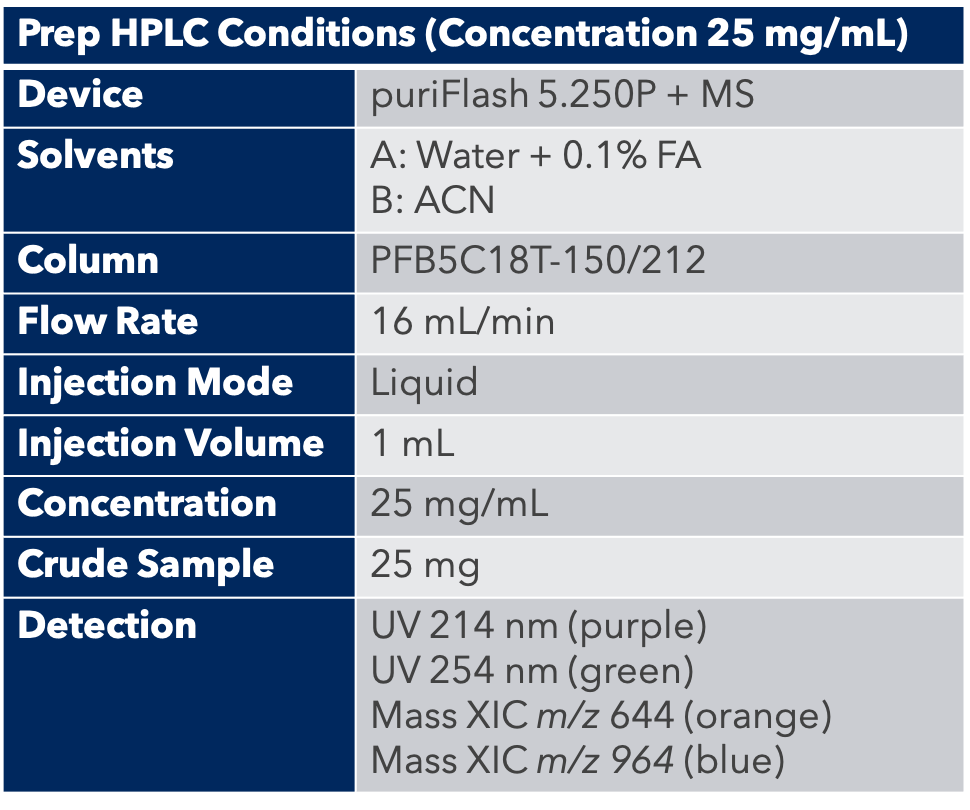
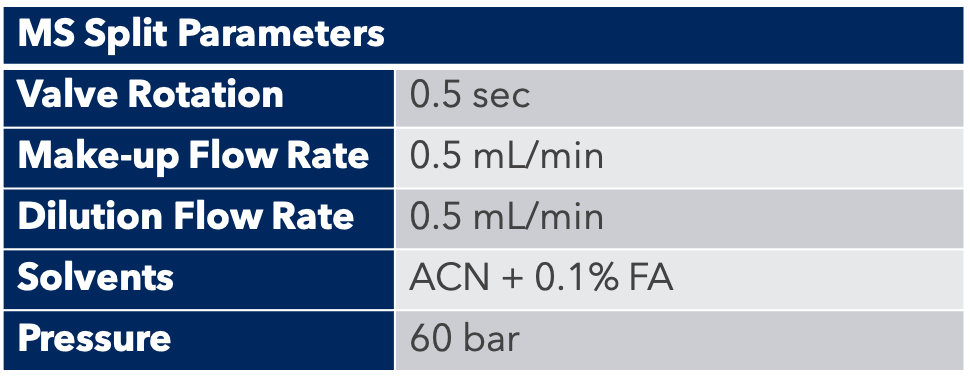
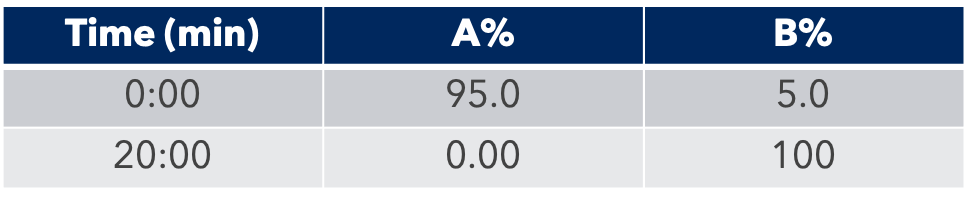
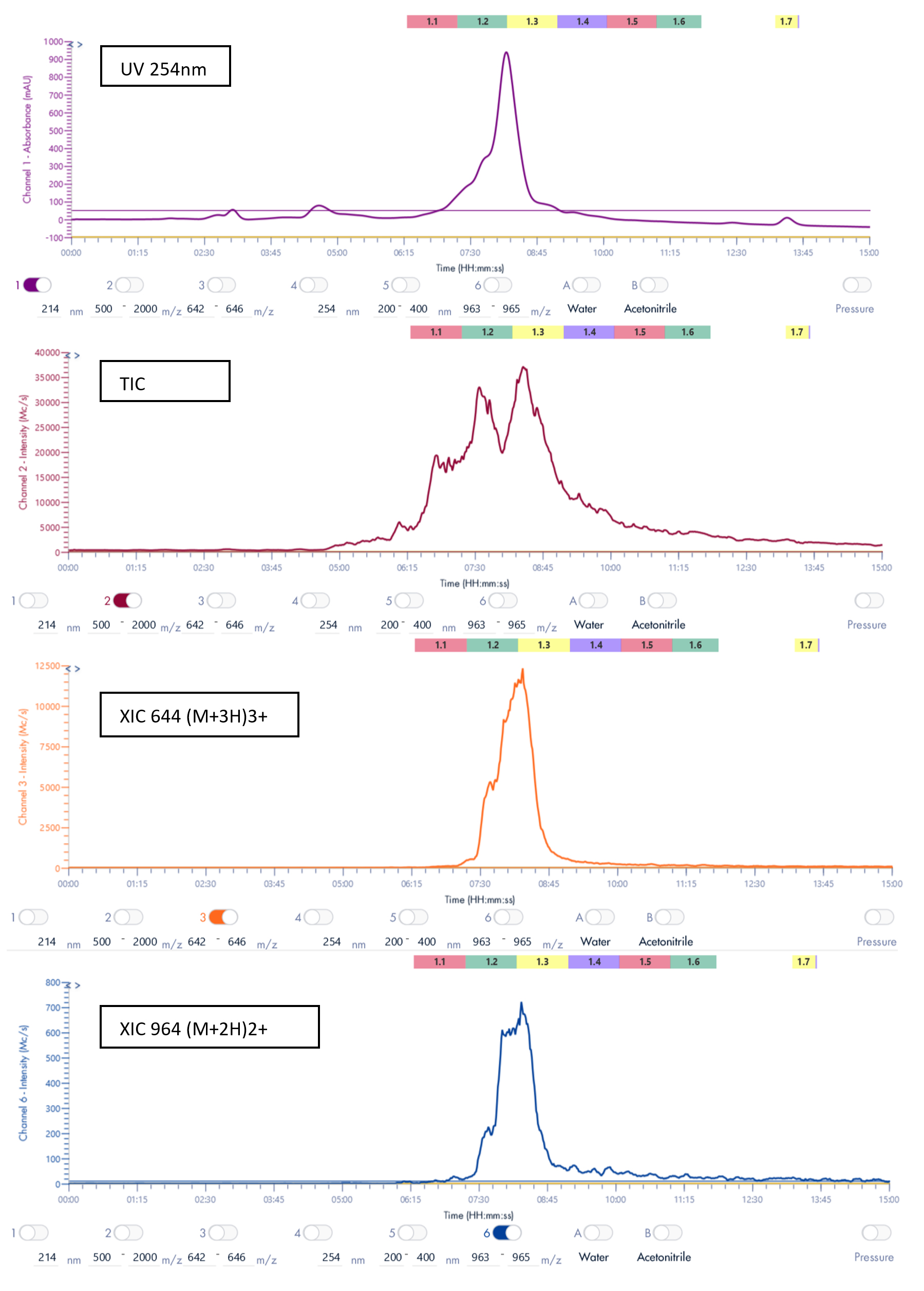 Thanks to the puriFlash 5.250P system coupled with the ex
Thanks to the puriFlash 5.250P system coupled with the ex